Revolutionizing the Removal of Ruptured Silicone Breast Implants
Introducing the First FDA-Cleared Device for a Focused Approach to Implant Removal
Tackling the Challenge of Implant Removal
Traditional methods of removing ruptured silicone breast implants can be complex and cumbersome. The lack of a specialized tool for this purpose has been a clear gap in procedural efficiency and precision. Recognizing this need, a new solution has emerged.
Did you know?
Breast augmentation remains one of the top five most common cosmetic procedures in the U.S., with more than 287,000 procedures performed annually. The majority of these, approximately 85%, involve silicone breast implants. Over time, the risk of implant rupture increases, reaching an average rate of 10% at 10 years post-surgery, and it is inevitable that all implants will eventually rupture.
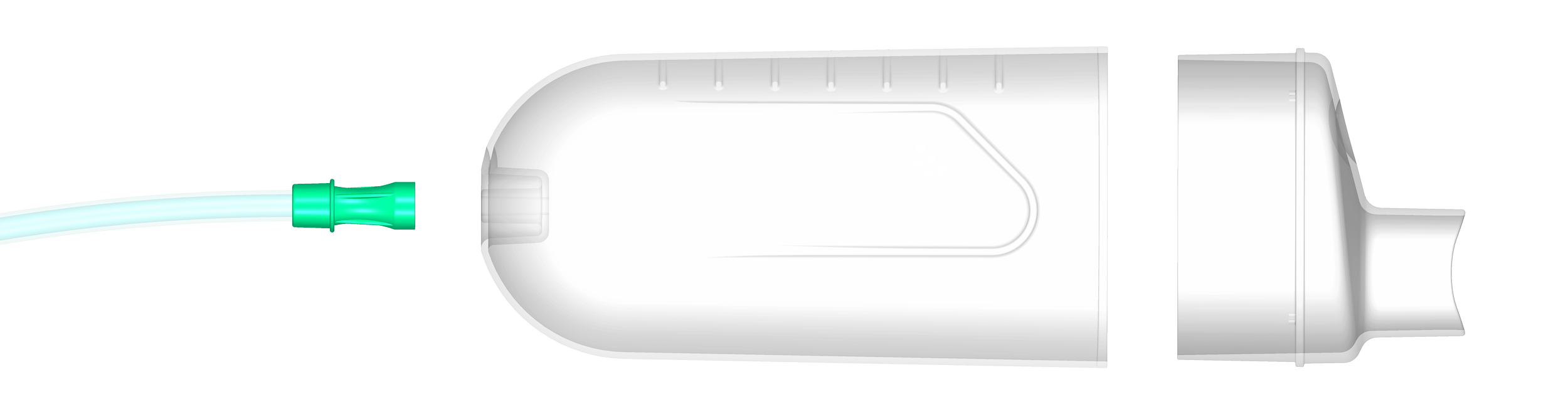
The Solution: The Breast Implant Removal Device (BIRD)
BIRD is a single-use, FDA-cleared suction device designed to assist in the removal of intracapsular ruptured silicone breast implants. It offers a targeted approach to streamline the removal process.
Designed with Compliance and Precision in Mind
BIRD is engineered for single-patient use and is specifically developed for the removal of intracapsular ruptured silicone breast implants. While not intended for en bloc removal or direct tissue contact, its design is focused on enhancing procedural efficiency within its intended use.
Want to learn the details? Download the Instructions For Use (IFU)
Need to order? Download the Order Form and email to sales@usethebird.com
Contact us.
sales@usethebird.com
(989) 448-8103
1258 Old 27 N.
Gaylord, MI 49735